Case REport 2301: SYSTEMIC INFECTION IN A DOMESTIC FERRET - DIAGNOSTIC DILEMMA 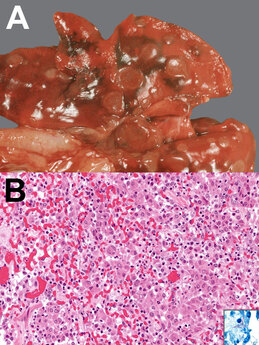 In October 2022, a dark-eyed white female spayed ferret experienced difficulty walking and breathing. The following Case Report is based on the owner's perseverance to uncover the cause of the ferret's condition. At the start of her clinical signs, the ferret was estimated to be around 5 years of age. When young she had been a rescue in the Los Angeles area. Since it is illegal to sell ferrets in California, absent commercial breeder identifiers (ear tattoos, etc.), the ferret was assumed to come from a local private breeder. By history the ferret took walks with her owner in the dusty Southwestern United States. Prior to the current illness, the ferret had been diagnosed with adrenal disease managed with hormonal implants. She was also being treated with an oral steroid drug, prednisolone, for symptomatic low blood glucose (hypoglycemia), which was likely due to insulinoma, a condition commonly found in domestic ferrets. Although she had a history of Aleutian’s disease, the AD virus was considered no longer active. On veterinary examination, the ferret was febrile with difficult breathing. Multiple lung masses were noted on radiologic examination, along with a pleural effusion (liquid in the lining of the lungs) and enlarged lymph nodes in the spleen and abdomen. She was considered a poor candidate for surgery, and surgical biopsies were not taken. Instead lymph aspirates were taken of the spleen and abdomen which showed increased cellular activity in the lymph notes, indicating either infection or inflammation, in the absence of lymphoma. Further testing also ruled out Coccidiomycosis (“Valley Fever”), a fungal infection. Over several months, the ferret was hospitalized twice for short-term courses of antibiotics (not described). Treatment was reported as "successful" in resolving the respiratory signs. Although she tolerated the treatment well, over time her condition continued to decline, and the owner made a decision to euthanize. On necropsy lymphadenopathy and monocytosis were reported. The report concluded that there was “multi-system pyogranulomatous disease affecting the kidneys, lymph nodes, spleen, and vessels most severely with milder disease in the liver and pancreas.” This condition can result from viral, fungal, and bacterial infection and, therefore, further testing was undertaken. Aleutian mink virus is known to cause multiorgan failure due to pyogranulomatous changes; however, this possibility was ruled out. Ferret systemic coronavirus was also eliminated. This was based on a coronaviral immunohistochemistry assay designed for feline mutated enteric coronavirus (also known as FIP) that targets specific viral proteins shared by both ferret and feline coronaviruses. Microscopic examination of the tissue lesions showed no evidence of fungal organisms, leaving bacteria as the presumptive cause of the infection. In-house aerobic culture of frozen renal tissue was reported out as “no bacterial growth after 48 hours” and also “no aerobic growth at [over] 5 days.” Routine testing includes the “acid fast” stain test, which should be positive in presence of mycobacteria (a group that includes tuberculosis). Further microscopic examination revealed “pyogranulomas with acid-fast stained, thin, linear, multifocally branching organisms suggestive of Nocardia.” Another candidate with similar microscopic appearance, Actinomyces, was eliminated due to the fact that it is “acid-fast” negative. Furthermore a polymerase chain reaction (PCR) assay for mycobacteria also proved negative. Tissue samples were cultured up to 14 days for the Nocardia bacteria did not return definitive results. A PCR assay would be next on the list, but this assay was not readily available at the institution. FINAL DIAGNOSIS When PCR was later performed, testing identified Mycobacterium celatum (MC), which, like Nocardia, has an acid-fast stain and is found in soil, waterways, and dust. COMMENTARY: MC is a slow growing potentially pathogenic mycobacterium. Initially described in 1993 in immunocompromised humans with the HIV-AIDS, MC infection has resulted in death in both immunocompetent and immunocompromised patients. Initial clinical presentation includes cough, malaise, and weight loss, associated with cavitary lesions and pulmonary infiltrates on xray. [Jun 2010] MC infection has also been previously reported in domestic ferrets. [Ludwig 2011, Piseddu 2011] Gross and histologic presentations from the lung tissue of a 3-year neutered male domestic ferret are shown in the following Figure. Figure: Gross (A) and histologic (B) appearance of lung tissue in a domestic male ferret infected with M. celatum [From Ludwig 2011; CDC.gov] A) Gross appearance: multiple, round light brown foci over lungs. B) Histologic appearance, granulomatous pneumonia: alveoli filled with foamy macrophages, epithelioid cells, and a multinucleated giant cell; also mild interstitial infiltration with lymphocytes, plasma cells, and neutrophils. Hematoxylin and eosin staining, original magnification x200. Inset, slender, rod-shaped, acid-fast bacilli in the cytoplasm of epithelioid cells; Ziehl-Neelsen staining, original magnification x400. Clinically relevant mycobacteria species in ferrets are M. genavense and M. microti, among others. In Europe, naturally occurring mycobacterial infections in ferrets are rare; but in New Zealand, M. bovis or M. avium complex infections in ferrets are common. [de Lisle 2008] This case appears to be the first MC infection documented in the United States in a domestic ferret. Diagnosis in the face of nonspecific clinical and radiographic findings was challenging. In addition, the laboratory and pathologic findings were compatible with numerous infectious pathogens of viral, fungal, and bacterial origin. Initial testing ruled out active viral infection due to ADV or to a coronavirus. Further testing ruled out Coccidiomycosis, and microscopic examination at postmortem further excluded the presence of fungal infection. Refocusing on a bacterial etiology, cultures and special stains were performed. Laboratory diagnosis of bacteria is based on microscopy and culture isolation. In this case, initial microscopic assessment showed: “pyogranulomas with acid-fast stained, thin, linear, multifocally branching organisms suggestive of Nocardia.” But Nocardia can be easily mistaken for Mycobacterium: not only does Norcardia grow in specific media for mycobacteria, but Nocardia also form partially acid-fast beaded branching filaments, similar to those formed by rapidly growing mycobacteria. From Muricy [2014]: “Change in cell morphology of Nocardia depending on the age of the culture may….lead to misidentification. Young cultures exhibit branched-long filaments, while old cultures present bacilli and cocci originating from fragmentation of filaments, which can be considered mycobacteria bacilli for non-expert microscopists.” In addition [Ibid.]: “Despite the Ziehl Neelsen method having been used for strong acid fast bacteria, such as the Mycobacterium species, and the modified Ziehl Neelsen method for weak acid fast bacteria, such as the Nocardia species, [in one study] Nocardia [was detected] by the Ziehl Neelsen method used for mycobacteria. This fact shows the importance of an expertise reading, since both genera can be detected.” Colony morphology from many species of Nocardia may be confused with mycobacteria, making diagnosis difficult. Moreover, microscopic analysis must be carefully performed by experienced technicians, as Nocardia can be misidentified as mycobacteria in the routine diagnosis of TB. In such cases, differentiation is performed by observation of aerial hyphae, produced only by species of the genus Nocardia. Diagnosis of Nocardia based on phenotype (appearance) alone can result misidentification. In a study comparing 6 isolates of Nocardia to Mycobacterium, only 2 were correctly identified by phenotypic identification. In such cases PCR and molecular species typing “by 16S rDNA sequencing seem to be essential for an early and definitive diagnosis.” [Ludwig 2011] In the case of this ferret, her history of comorbidities (adrenal and pancreatic cancers), as well as chronic steroid treatment likely resulted in her being immunosuppressed. Correct identification of the causal agent is very important, as it informs the correct course of therapy. Mycobacteria respond to different drugs from those used to treat Nocardia. Even so, “mixed” infection – cases in which both Nocardia and mycobacteria species were identified--have been found in immunocompetent patients. [Muricy 2014] Therefore, in the case of treatment failure, further bacteriological investigation is warranted. [Link to PDF] REFERENCES:
Comments are closed.
|
Details
Archives
April 2024
Categories
All
|
|
Terms and Conditions
|
allFerrets® 2014-2024. ALL RIGHTS RESERVED.
Proudly designed by widgIT |


 RSS Feed
RSS Feed
